Ahmedabad
(Head Office)Address : 506, 3rd EYE THREE (III), Opp. Induben Khakhrawala, Girish Cold Drink Cross Road, CG Road, Navrangpura, Ahmedabad, 380009.
Mobile : 8469231587 / 9586028957
Telephone : 079-40098991
E-mail: dics.upsc@gmail.com
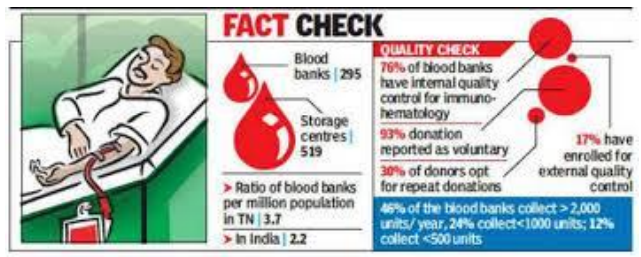
Summary of Key Highlights • Regulatory Overhaul: The Union government has introduced stringent regulations and raised entry barriers for establishing blood centres to eliminate \'professional\' and \'replacement\' donation cultures.• Eligibility Restructuring: Only registered voluntary and charitable organizations are now eligible to set up blood banks; family-run societies and private trusts are barred to prevent commercialization. • Infrastructure Mandates: New and existing centres must establish independent component separation and storage facilities within two years of receiving a permit, shifting away from simple storage to complex processing. • Audit and Compliance: Following incidents where children tested HIV positive post-transfusion, a nationwide audit of 4,153 licensed blood banks has been ordered by the Central Drugs Standard Control Organisation (CDSCO). • Focus on 100% Voluntary Donation: The National Blood Transfusion Council (NBTC) aims to transition to a fully voluntary, non-remunerated system, which is statistically proven to have the lowest risk of Transfusion-Transmitted Infections (TTIs). • Operational Benchmarks: Organizations must now achieve an annual collection of over 2,000 units, with nearly 100% sourced from voluntary donors, to maintain their license. Key Definitions • Professional Donor: An individual who donates blood in exchange for money or other commercial benefits, a practice legally banned in India since 1998 due to high infection risks. • Replacement Donation: A system where the family or friends of a patient are required to provide a donor to \'replace\' the units used by the patient, often leading to coercion or hidden \'paid\' donors. • Component Separation: The process of splitting whole blood into various parts—Red Blood Cells (RBCs), Plasma, and Platelets—to treat multiple patients from a single donation. Constitutional & Legal Provisions • Article 21: The Supreme Court has linked the right to safe blood transfusion to the \'Right to Life,\' noting that medical negligence leading to infection is a violation of fundamental rights. • Drugs and Cosmetics Act, 1940: Human blood is legally categorized as a \'drug\' under Section 3(b). Operations of blood banks are governed under the Drugs and Cosmetics Rules, 1945. • Supreme Court Directive (1996): In Common Cause vs. Union of India, the Court ordered the abolition of professional blood donation and the establishment of the National and State Blood Transfusion Councils. • National Blood Transfusion Bill, 2025 (Proposed): A new legislative framework aiming to centralize oversight and set uniform national standards for blood safety and patient recourse. India\'s Blood Transfusion Landscape

Important Keypoints for UPSC • Public Health Priority: The shift from \'replacement\' to \'voluntary\' donation is a critical pillar of Universal Health Coverage (UHC), reducing the out-of-pocket burden and infection risks for marginalized patients. • Clustering vs. Accessibility: New norms mandate at least one blood bank per district while discouraging over-clustering in urban areas to ensure equitable rural access. • Quality Assurance: The CDSCO and the Drug Controller General of India (DCGI) are strengthening the No-Objection Certificate (NOC) process through State Blood Transfusion Councils. • Technical Gap: India still faces a shortage of O-negative blood and AB-positive plasma; the new infrastructure mandates for component separation aim to optimize these rare resources. Conclusion The government\'s crackdown on unethical donor practices represents a transition from a supply-driven to a safety-driven blood transfusion ecosystem. By professionalizing the management of blood centres and enforcing strict social accountability, the state seeks to rebuild public trust in the healthcare system, ensuring that the \'gift of life\' does not inadvertently become a source of life-threatening infection. UPSC Relevance • GS Paper II: Issues relating to development and management of Social Sector/Services relating to Health; Role of regulatory bodies (CDSCO, NBTC). • GS Paper III: Science and Technology- developments and their applications in everyday life (Transfusion medicine); Challenges to internal security (Safe medical infrastructure). • Prelims: Mandatory tests for blood, legal status of blood as a \'drug,\' and functions of the NBTC/CDSCO.
Address : 506, 3rd EYE THREE (III), Opp. Induben Khakhrawala, Girish Cold Drink Cross Road, CG Road, Navrangpura, Ahmedabad, 380009.
Mobile : 8469231587 / 9586028957
Telephone : 079-40098991
E-mail: dics.upsc@gmail.com
Address: A-306, The Landmark, Urjanagar-1, Opp. Spicy Street, Kudasan – Por Road, Kudasan, Gandhinagar – 382421
Mobile : 9723832444 / 9723932444
E-mail: dics.gnagar@gmail.com
Address: 2nd Floor, 9 Shivali Society, L&T Circle, opp. Ratri Bazar, Karelibaugh, Vadodara, 390018
Mobile : 9725692037 / 9725692054
E-mail: dics.vadodara@gmail.com
Address: 403, Raj Victoria, Opp. Pal Walkway, Near Galaxy Circle, Pal, Surat-394510
Mobile : 8401031583 / 8401031587
E-mail: dics.surat@gmail.com
Address: 303,305 K 158 Complex Above Magson, Sindhubhavan Road Ahmedabad-380059
Mobile : 9974751177 / 8469231587
E-mail: dicssbr@gmail.com
Address: 57/17, 2nd Floor, Old Rajinder Nagar Market, Bada Bazaar Marg, Delhi-60
Mobile : 9104830862 / 9104830865
E-mail: dics.newdelhi@gmail.com